

of patients with DED have excessive tear evaporation associated with meibomian gland dysfunction (MGD)3-5
Clinic-based cohort studies have shown


of patients with DED have excessive tear evaporation associated with meibomian gland dysfunction (MGD)3-5



I wish my eye doctor spent more time explaining what causes dry eye.

*Survey sponsored by Bausch + Lomb was conducted by The Harris Poll amongst N = 732 US “dry eye sufferers” (those who often/always experience eye dryness and/or have been diagnosed with DED).6
MGD is characterized by:
Changes in meibum composition8
Reduced meibum secretion9
Breakdown of the tear film lipid layer2,8
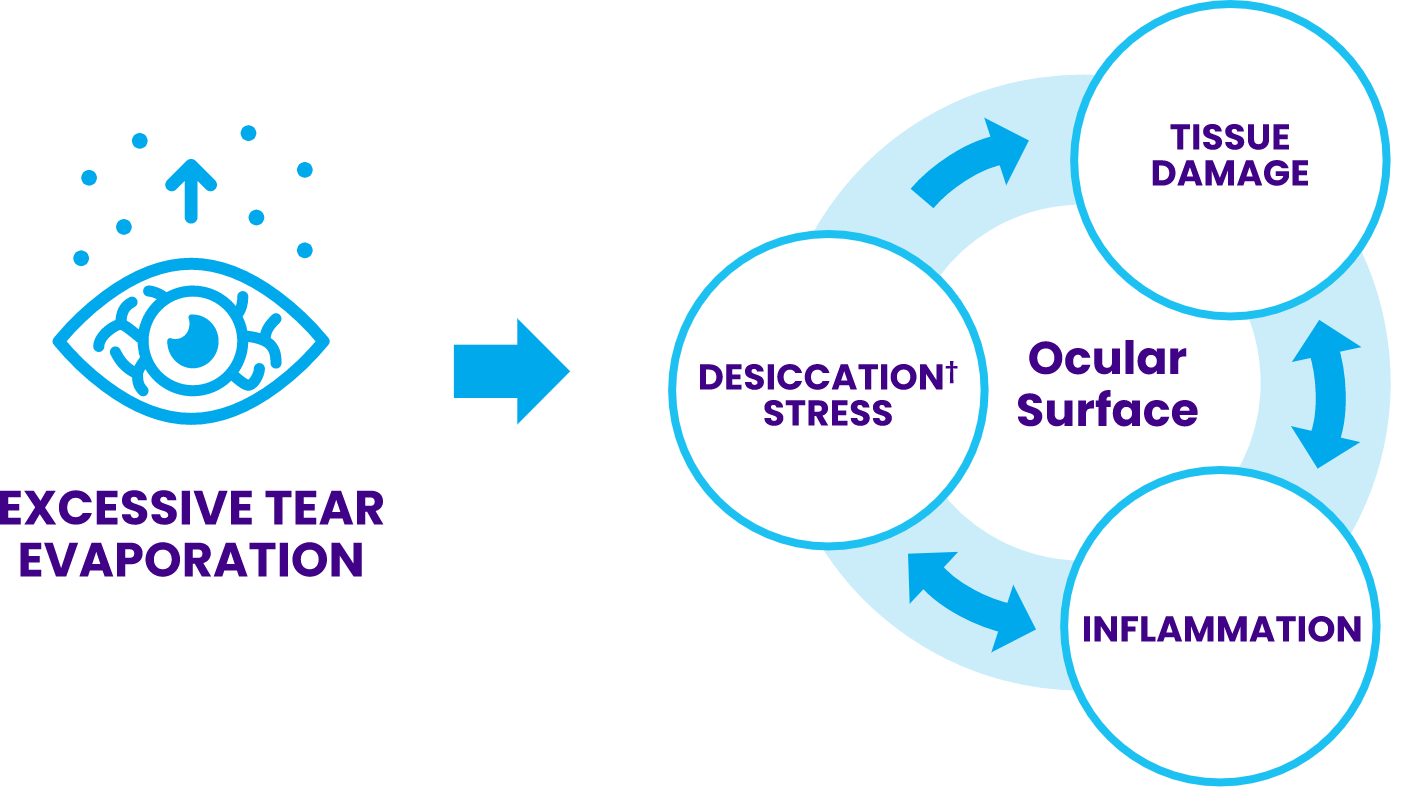
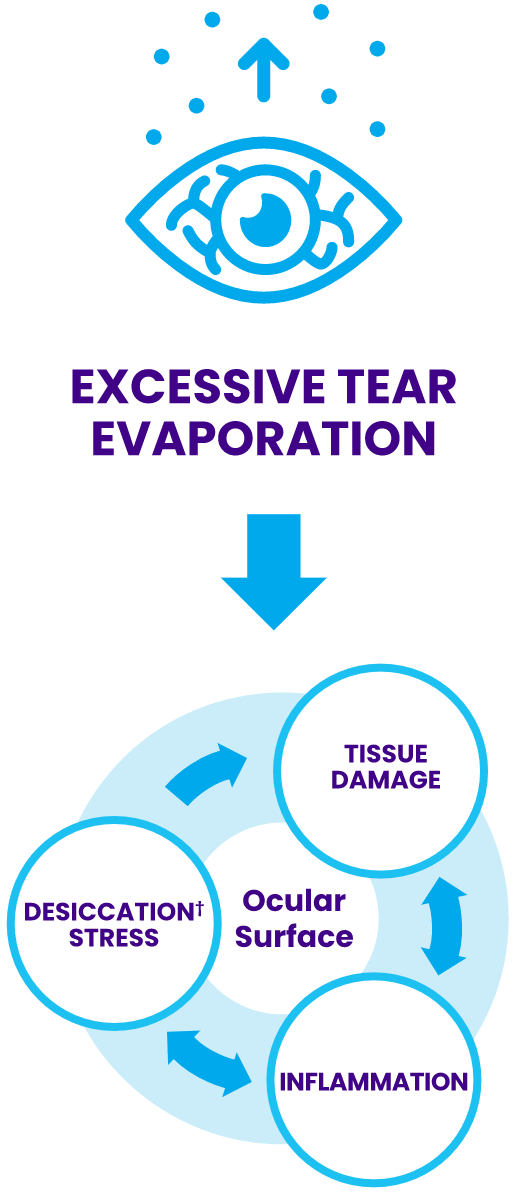
†Desiccation is the drying of the ocular surface due to tear evaporation exceeding tear production.

reported an increase in patients aged 18-34 presenting DED symptoms

indicated that the use of technology contributes to DED symptoms

agreed that DED is becoming more common due to a multiscreen lifestyle

Learn more from the TFOS Lifestyle Epidemic Report about the impact of today’s world on DED
DED, dry eye disease; TFOS, Tear Film & Ocular Surface Society.
Successful treatment of dry eye should be aimed at restoring the homeostasis of the ocular surface microenvironment.”
—American Academy of Ophthalmology Dry Eye Syndrome Preferred Practice Pattern® guidelines12

Most leave excessive evaporation unaddressed—as a result, appropriate management of evaporative DED may require an Rx option that directly targets tear evaporation13-19
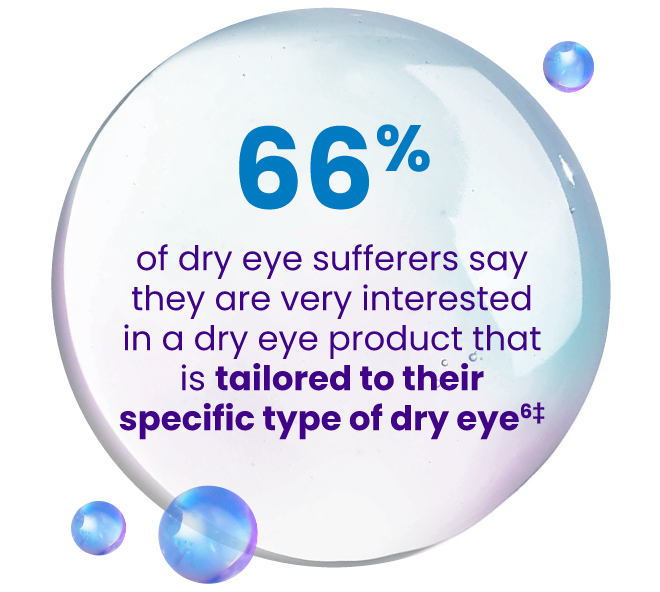
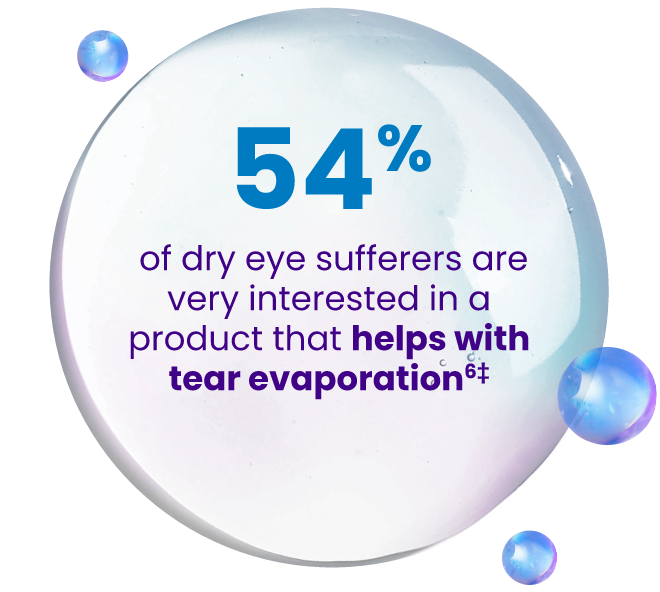


‡Survey sponsored by Bausch + Lomb was conducted by The Harris Poll amongst N = 732 US “dry eye sufferers” (those who often/always experience eye dryness and/or have been diagnosed with DED).6


Understanding Dry Eye Disease and Diagnosis With Dr. Marc Bloomenstein and Patient Rahnee
MIEBO® (perfluorohexyloctane ophthalmic solution) is indicated for the treatment of the signs and symptoms of dry eye disease.
Click here for full Prescribing Information for MIEBO.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
References: 1. Sheppard JD, Nichols KK. Dry eye disease associated with meibomian gland dysfunction: focus on tear film characteristics and the therapeutic landscape. Ophthalmol Ther. 2023;12(3):1397-1418. doi:10.1007/s40123-023-00669-1 2. Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II report executive summary. Ocul Surf. 2017;15(4):802-812. doi:10.1016/j.jtos.2017.08.003 3. Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472-478. doi:10.1097/ICO.0b013e318225415a 4. Rabensteiner DF, Aminfar H, Boldin I, et al. The prevalence of meibomian gland dysfunction, tear film and ocular surface parameters in an Austrian dry eye clinic population. Acta Ophthalmol. 2018;96:e707-e711. doi:10.1111/aos.13732 5. Badian RA, Utheim TP, Chen X, et al. Meibomian gland dysfunction is highly prevalent among first-time visitors at a Norwegian dry eye specialist clinic. Sci Rep. 2021;11(23412):1-8. doi:10.1038/s41598-021-02738-6 6. Bausch + Lomb 2025 State of Dry Eye Survey. 7. Al-Mohtaseb Z, Schachter S, Shen Lee B, Garlich J, Trattler W. The relationship between dry eye disease and digital screen use. Clin Ophthalmol. 2021;15:3811-3820. doi:10.2147/OPTH.S321591 8. Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438-510. doi:10.1016/j.jtos.2017.05.011 9. Yeotikar NS, Zhu H, Markoulli M, Nichols KK, Naduvilath T, Papas EB. Functional and morphologic changes of meibomian glands in an asymptomatic adult population. Invest Ophthalmol Vis Sci. 2016;57(10):3996-4007. doi:10.1167/iovs.15-18467 10. Zhang R, Pandzic E, Park M, Wakefield D, Di Girolamo N. Inducing dry eye disease using a custom engineered desiccation system: impact on the ocular surface including keratin-14-positive limbal epithelial stem cells. Ocul Surf. 2021;21:145-159. doi:10.1016/j.jtos.2021.04.006 11. Modern technology and a multi-screen lifestyle viewed as Important factors in rising prevalence of dry eye disease. PR Newswire. Published October 17, 2016. Accessed September 8, 2025. https://www.prnewswire.com/news-releases/modern-technology-and-a-multi-screen-lifestyle-viewed-as-important-factors-in-rising-prevalence-of-dry-eye-disease-597289311.html 12. Amescua G, Ahmad S, Cheung AY, et al; American Academy of Ophthalmology Preferred Practice Pattern Cornea/External Disease Panel. Dry Eye Syndrome Preferred Practice Pattern®. Ophthalmology. 2024;131(4):PP1-P49. doi:10.1016/j.ophtha.2023.12.041 13. Cequa. Prescribing Information. Sun Pharmaceutical Industries, Inc. 14. Eysuvis. Prescribing Information. Alcon Laboratories, Inc. 15. Restasis. Prescribing Information. AbbVie Inc. 16. Tyrvaya. Prescribing Information. Oyster Point Pharma, Inc. 17. Vevye. Prescribing Information. Harrow Eye, LLC. 18. Tryptyr. Prescribing Information. Alcon Laboratories, Inc. 19. MIEBO. Prescribing Information. Bausch & Lomb, Inc.
MIEBO® (perfluorohexyloctane ophthalmic solution) is indicated for the treatment of the signs and symptoms of dry eye disease.
Click here for full Prescribing Information for MIEBO.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
FAQs